During the manufacturing of metal parts, their surface aspect is a very important parameter as it defines the cosmetic aspect, the resistance to external aggression and the potential friction they may have while in contact of other components. Surface optimization is called surface treatment, these treatments can be mechanical, chemical or done by deposition of external material.
Mechanical surface treatment
In this category includes processes of metal part surface reworked with a mechanical contact.
Micro peening / Sandblasting / Shot blasting
These three processes are rather similar and consist in high speed propulsion of particules on all or part of the surface of part. The particules differs in kind and size, respectively : microbeads ,sand grain and hard steel shots. Impact of the particules on the metal surface will reinforce the material surface (by local compression), enabling impurities and corrosion stain removal and create an homogeneous aspect with grain size related to the blasting particules used. These surface treatment processes are also used for preparation of surface before painting application by improving surface grip.

Micro peening aspect – AGC IS
Tribofinishing
Also called barrel finishing, it consist in putting in contact in a device parts to be treated and abrasive mixture. Tribofinishing device will emit repetitive moves in order to create vibration and then friction between the parts surface and the abrasive mixture. These friction will create a smoothing of the part aspect, break sharp angles, clean surfaces of impurities and create a homogeneous aspect.
Polishing / Brushing
Brushing and polishing are processes of friction between an abrasive and metal surfaces that renders a homogeneous aspect of the surface. Brushing will leave characteristic lengthy marks while the objective of polishing will be not to leave any visible processing mark on the surface. Metal polished surface may have a functional use as a light reflector/ mirror. Brushing is mainly used for cosmetic means.

Brushed metal – AGC IS
Chemical surface treatment
Chemical surface treatment allows the modification of surfaces at the molecular level by the action of external chemical agents and catalysts.
Anodisation
Anodisation is a process for the surface treatment of aluminium which consists of a controlled oxidation of aluminium parts surface. This oxydation takes place in a catalytic bath where the O2- ions interact with the aluminium to create a layer of alumina. This very hard layer provides good mechanical, chemical and corrosion protection for the parts.
This very effective process improves the appearance of the parts and by adding chemical dyes to the anodising baths can be coloured on request. Finally, anodisation also permits the electrical insulation of parts, whereas pure aluminium is a very good conductor.
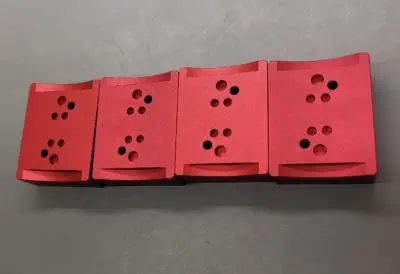
Red anodisation on machine aluminium parts – AGC IS
Passivation
Stainless steel is not very prone to corrosion, but it is not entirely free of the risk, particularly because of certain impurities that can be embedded on the surface. Passivation will clean the surface of stainless steel parts, parts are dipped in a heated acid solution and remain immersed for several tens of minutes, then rinsed to remove the acid solution. The parts are strengthened against corrosion by the removal of impurities and the creation of a very thin layer of chromium oxide on the surface during the process.
Burnishing / Blackening
The process consists of dipping steel, copper or brass parts in acidic chemical baths doped with Copper (Cu) and Selenium (Se). Burnishing surface treatment allows the appearance of a homogeneous layer of black color allowing a better resistance to corrosion, a pleasant aspect and a lesser reflection of the light (useful for optical applications).

Burnished metal part – AGC IS
Surface treatment by material layer deposition.
As most metals are subject to corrosion, coating them with other materials/alloys may improves their resistance to external elements.
Painting
Application of paint to protect metal parts is the cheapest and therefore the most widely used method, especially for construction. It can be applied by brush, dip or spray, commonly on dedicated painting lines.
As well as being quick to apply and inexpensive, paint also allows access to a wide range of colors and the possibility of touching up. However, paint will tend to flake over time, so it must be reapplied regularly.

Metal painting: the Eiffel Tower
Cataphoresis
Cataphoresis is an advanced painting process that consists of immersing parts in a water-soluble paint bath, while the deposition of the paint layer will be controlled by an electric field. This process allows for a better resistance of the paint compared to the application methods listed above, and can resist salt spray test for 720h and more.
In addition, paint is distributed very evenly over all parts of the part to be treated, the control of the bath time allows a very good control of the paint layer thickness which can therefore be applied even on threads. This technique is also referred to as electroplating.
Furthermore, cataphoresis limits paint losses because roughly all the paint dissolved in the treatment bath is used.
Common aspect is a beautiful black (also exists in white) with a thickness of a few tens of µm.
Galvanization
Hot-dip galvanizing is the process of protecting steel by applying a protective layer of zinc. The main purpose of galvanization is to protect against corrosion. This application is carried out by putting the steel parts to be treated through a liquid zinc bath (450°C). The bond between the steel and the zinc is a strong molecular bond that offers a very resistant grip to mechanical friction.
The zinc layer deposited is from several tens of microns to more than 100 microns thick, depending on the level of protection required. A hot-dip galvanizing layer effectively protects the steel for decades. Traffic signal posts on roads are notably protected by hot-dip galvanizing, this treatment is particularly prized for structural parts.
The interest of hot dip galvanizing is that the zinc layer offers a “healing” capacity, if the surface is scratched, the protective layer will recreate itself and avoid the corrosion of the steel.
Nickel / Chrome plating
Electroplating of a layer of Nickel and/or Chromium. These two surface treatments improve the appearance by increasing the brightness and provide good protection against metal corrosion.
Coatings are usually a few microns thick (cosmetic use only) to a few hundred microns thick (hard chrome plating) for corrosion protection and mechanical strengthening of treated metal parts.
Metals treated are steel, copper and brass. For better adhesion and protection against corrosion, the application of chromium is often preceded by a layer of nickel. Hard chromium plating is particularly appreciated for its high resistance to friction in the production of pistons.
It should be noted that the chromium plating process must no longer be carried out with hexavalent chromium but with trivalent chromium in order to comply with the European RoHS directive aimed at better protection of the environment.

Nickel plated steel structure – AGC IS
Tinning
Tinning is a process of depositing a layer of a few microns of tin on metals. This very old surface treatment process (dated back to antiquity) is currently mainly used in Electronics/Electricity for the protection against oxidation of copper connections while offering a very good solderability (brazing).








